![]() Download Newsletter in PDF format
Download Newsletter in PDF format
 From the start, iConquerMS™ has been an initiative that’s all about research. Our “north star” is to drive research of interest to people with MS on topics they care about, as well as to create a valuable national MS database that can power future research studies.
From the start, iConquerMS™ has been an initiative that’s all about research. Our “north star” is to drive research of interest to people with MS on topics they care about, as well as to create a valuable national MS database that can power future research studies.
As you may know, iConquerMS™ is funded by the nonprofit called PCORI (Patient-Centered Outcomes Research Institute), which is dedicated to building an evidence base for the nation’s healthcare. PCORI has an intense interest in MS, reflected in their announcement that it is making up to $50 million available to fund randomized clinical trials or large observational studies that compare two or more forms of therapy for MS. The funding announcement resulted from a series of stakeholder meetings in which Laura Kolaczkowski, our co-Principal Investigator, Joe LaFerrera, a member of our Governing Board, and I participated as representatives of iConquerMS™. As the only PCORI-funded MS Patient-Powered Research Network, iConquerMS™ is well positioned to contribute significantly to such studies through our expertise, our growing body of participants, and the guiding hand of our volunteer leadership.
Less than a year after our formal launch in February 2015, I’m thrilled to report that we are making headway along four simultaneous tracks:
1) iConquerMS™ participation. At last count, almost 2700 individuals from across the country have become fully consented participants in iConquerMS™, with the majority submitting health data to the network. Not surprisingly, volunteers from the MS community have been the greatest driver of recruitment, through blogs and other social media that endorse our efforts. In the coming year, we plan to double our cadre of participants.
2) Engagement of people with MS in research concepts and design. iConquerMS™ has multiple opportunities for those living with MS to express their views about research. As part of the governance structure, iConquerMS™ has a standing Research Committee (co-chaired by Hollie Schmidt, MS, and Deborah Backus, PhD – see an interview with Debbie, below) comprised of many research experts including people with MS. All research ideas are reviewed by that group for importance and suitability. Engagement of the entire iConquerMS™ community is also an essential part of the research development process. As iConquerMS™ studies are designed and developed, participants are asked to indicate their level of interest in the research topic and to comment on the study design including whether it would be feasible to participate in. (See the article below about the mood treatment study as an example of this type of engagement.) And this fall, with financial assistance from Genentech, we conducted four ‘Research Studios’, inviting people with MS to meet in person with researchers and physicians to comment on their research interests and priorities (see article below).
3) Research project grant submissions. iConquerMS™ has submitted a full technical proposal to PCORI for a Large Pragmatic Clinical Study to compare the effectiveness of various MS treatments. We also submitted a full proposal in collaboration with the MoodNetwork PPRN for a study comparing two types of Internet-based mental health treatments tailored for people with MS. In addition, iConquerMS™ has contributed to 7 Letters of Intent (LOIs) for PCORI funding and one funding application sent to the National MS Society. We are hopeful that several of these proposals will be funded and look forward to engaging iConquerMS™ participants in the studies that follow.
4) Research outcomes dissemination. A central mission of iConquerMS™ is that research results should be shared as broadly as possible among people with MS, researchers and clinicians. We have begun this task by sharing “comparative” data about our network (age, gender, etc) with our participants at the registered section of the iConquerMS™ portal, as well as the results of the online surveys we’ve conducted (see article below). As soon as planned research studies reach completion, we will share those results as well. In addition, we have developed a special section of the iConquerMS.org portal especially for researchers, so that they can both learn about our resources for research and be stimulated to develop new research proposals of interest to people with MS (see https://iconquerms.org/for-researchers).
In addition to the activities described in this newsletter, iConquerMS™ is also building bridges within and outside our community:
- In January 2016, we will conduct our Second Annual Leadership Summit with sponsorship from Biogen.
- We attended the 2015 ECTRIMS conference, the largest annual meeting of researchers and clinicians in the field of MS, and shared iConquerMS™ updates with attendees.
- We attended the National MS Society Leadership conference, and shared iConquerMS™ with Society leaders, volunteers and staff in a special session on “Accelerating Research.”
Much more is planned in the coming months for our unique initiative. We welcome your ongoing input, your leadership, your ideas and your suggestions for how we can engage you, your family, friends and colleagues most effectively. And once again, we express our deepest appreciation for everything you are doing in the fight against MS.
Best wishes for a happy holiday season and an empowered New Year!
Sincerely,
Robert McBurney, Ph.D.
Principal Investigator, MS Patient-Powered Research Network (iConquerMS™)
President & CEO, Accelerated Cure Project for MS
Research “Studios” Build Bridge between People with MS and Researchers
Among the many roles played by iConquerMS™ in accelerating and enhancing MS research is to amplify the voices of people with MS about what’s important to them. This fall, iConquerMS™ organized four gatherings - in Atlanta, Denver and San Diego - that brought together people with MS and clinical researchers to surface research topics.
What’s the big idea? Simple, but revolutionary: to create novel ways for people with MS, caregivers, clinicians and researchers to talk with each other about disease-specific research.
Common themes raised by the participants during these gatherings included studies about possible causes such as genetics, viruses and Vitamin D deficiency; treatments such as stem cell transplants, medical marijuana and the recently announced ocrelizumab (among many other disease modifying therapies); and the impact of self-care options in the areas of exercise, diet and stress management. In several meetings, participants cited the increase over the past decade in the number of African- Americans diagnosed with MS and noted that research on MS in African-Americans lags behind studies involving Caucasian subjects. Numerous participants expressed the belief that they had had MS “for a long time” before being correctly diagnosed with the disease.
In Atlanta, topics relating to physical therapy, exercise and stress management took center stage, with several participants expressing a desire to push themselves to their maximum capability, in the hope of regaining some portion of the athleticism and the cognitive abilities they possessed before contracting MS. They raised the question of whether it’s possible to reverse damage and disability through physical exercise and purposeful mental activity.
People with MS in the Denver meeting proposed research questions having to do with heredity, aging, fatigue and cognitive decline, wondering whether methods of measuring progress could be tailored to a patient’s particular strengths and weaknesses. Some wanted research into the possible positive impacts of yoga, meditation, diet and acupuncture. Others asked about studying the genetic biomarkers of responses to various MS treatments. One neurologist asked for participants’ opinions about the possibility of conducting neurological exams via Skype, to make drug safety monitoring visits more convenient. He noted that computerized assessments could also provide consistent, objective measurement of functions.
Topics that interested participants in San Diego were the impacts and efficacy of autologous stem-cell transplants and the use of medical marijuana (only legally available in a handful of states).
In all four gatherings, participants expressed frustration with the challenges of determining which disease modifying therapy is best for them. Said one, “I’ve made a flowchart for myself based on my own knowledge, but I would be glad to have outside input on this. Can’t we bring all of the clinical trial data together to see which is the best drug?” (Note: that is exactly the purpose of the Large Pragmatic Clinical Study submitted for funding by iConquerMS™!)
Additional Research Studios are planned for 2016, as part of the ‘two-way traffic’ across the bridge between researchers and people with MS. We also plan to hold some studios via webcast to expand participation.
Mood Treatment Study Proposal: How the iConquerMS™ Community Shapes Research
Over the summer of 2015, the iConquerMS™ community was invited to share their thoughts on depression and other mood issues. This input was used to develop a funding proposal for a study comparing two types of Internet-based mental health treatments for people with MS.
Over 400 iConquerMS™ members responded to each of the two online surveys. Using that input, the iConquerMS™ team worked with the MoodNetwork PPRN that is associated with the Massachusetts General Hospital and other colleagues to develop the proposal, submitted to PCORI in October.
iConquerMS™ members were asked to complete two surveys, one about mood issues and treatment, and the other about the study design and goals. The results - gathered with remarkable speed and efficiency - were illuminating:
Impact of mood issues and treatment experience:
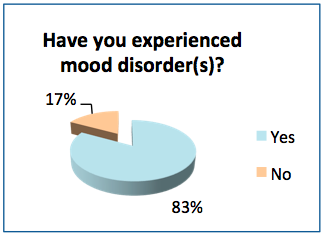
- 83% of the respondents have had mood issues at some point in their lifetime, and 55% have received a medical diagnosis for a mood disorder.
- Respondents reported use of several types of treatments:
- Medication: 63%
- Mindfulness: 22%
- Cognitive behavioral therapy (CBT): 20%
- Peer support: 16%
- Other talk therapy: 26%
- Acceptance and commitment therapy: 4%
However, 26% have never received any mood disorder treatment.
- Most people (73-83%) said they would be or might be interested in participating in a study involving CBT, mindfulness, peer support, or exercise.
- 46% of the respondents have received mental health care from their primary care provider, 38% from a neurologist, 38% from a psychologist or psychiatrist, and 24% from a social worker/counselor. (The totals exceed 100% because respondents could select more than one choice.) 11% said they have had problems getting access to mental health care in the past.
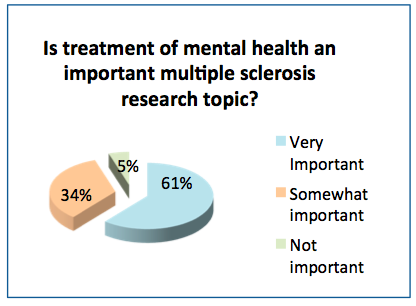
- 61% of the respondents felt mental health is a very important research topic for MS and 34% considered it somewhat important.
People were also asked to indicate what they wish was known about depression in MS. Some of the responses included:
 - What causes depression in people with MS?
- What causes depression in people with MS?
- How much of it is due to effects of the disease on the brain and how much is due to having MS?
- What treatments are helpful?
- How are depression and fatigue related? and
- Does depression promote relapses?
Study Design:
- We asked people to rate the importance of the following study goals:
- Learn more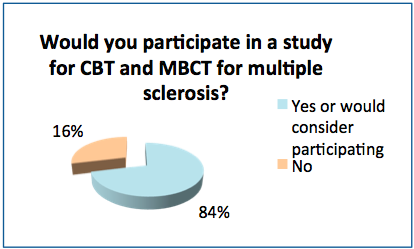 about how mood disorders are the same or different in people with MS compared with others
about how mood disorders are the same or different in people with MS compared with others
- Compare how cognitive behavioral therapy (CBT) and mindfulness-based cognitive therapy (MBCT) work in people with MS when delivered online.
- Determine whether either or both therapies work better in certain groups of people than in others.
- Communicate the results of the study to people with MS, their families, health care providers, and others.
- 71-85% of the respondents rated each goal as a 4 or 5 on a scale from 1 to 5.
- After reading a short description of the study comparing CBT and MBCT, 84% of the respondents said they would or might want to participate in it.
- Most respondents (62-84%) felt that it would not be a burden for them to participate in treatment for 8 weeks, spend 30-40 minutes per week in online treatment sessions, or fill out questionnaires during and after the treatment period.
- 72% of the respondents were concerned they might face barriers (such as cost) in getting access to an Internet-based therapy outside of a research study.

Using the survey responses from iConquerMS™ members and working with the Mood Network, another PCORI-funded patient-powered research network, iConquerMS™ submitted a full application to PCORI in October. Below is the public abstract summarizing the study goals and activities.
The study will be conducted in two phases. In Phase 1, we will recruit and enroll 1,000 people with MS from the iConquerMS™ PPRN, and 1,000 people from the MoodNetwork PPRN who have a mood disorder but do not have MS or another autoimmune or chronic medical condition. Participants will complete online surveys about their neurological status, general health and emotional well-being. We will use data from these surveys to compare the features and symptoms of depression and anxiety between the two cohorts. The results of these analyses could lead to improved screening and diagnosis of mood disorders in MS, as well as better monitoring of these conditions and improved use of the available treatments.

Spotlight on iConquerMS™ Governance:
Deborah Backus, Co-chair, Research Committee
Q: Your background seems to encompass an unusual mix of disciplines and skills. What’s the origin of your interest in MS research, and how has it evolved over the years?
Debbie: I started as a physical therapist, treating people with MS and spinal cord injury, and found that I wanted to answer questions about how to treat people with neurological disorders. Back then, there were no clinical facilities with research – that’s hard to believe, isn’t it? -- so I returned to school for my PhD in neuroscience, assuming I’d stay in academe. Fortunately, Shepherd Center has a research institute, and I got engaged in clinical research and integrating research into practice. When Shepherd got funding for MS rehabilitation research, I grew that program so that we can build and integrate research evidence into care in the Shepherd MS Institute. So, I see the combination of disciplines as a natural and logical way to improve care both for individual patients and the healthcare system in general.
Shepherd serves as a kind of ‘one-stop shop’ in spinal cord injury, brain injury and MS, since it offers treatment of acute neurological injury or disease; outpatient rehabilitation, and wellness (for MS this includes rehabilitation, exercise, cognitive training, social training, diet/nutrition, etc.) as well as research opportunities for people with MS. I think that a comprehensive, holistic approach in one center is unusual in the MS field, and it’s central to my work.
Q: Would you describe some of the highlights of your research in recent years, and what insights you’ve been able to garner?
Debbie: Much of my research is focused on how to expand the options that people with MS have in the context of where they are. For example, in one study we are evaluating an electrical stimulation tool used for spinal cord injury, in people with progressive MS who are in wheelchairs, as a potential way to overcome fatigue and improve overall quality of life. We found that almost everyone in the study could activate their leg muscles to cycle, and almost everyone improved their bike performance. This suggests that they have potential to improve – even though they have a chronic disease. So, I like to look for ways to empower people with MS to make significant improvements, as early as possible in their journey, and not wait until they worsen and have to react to disability.
Q: What do you see as the major challenges in MS research today? Funding? Data? Critical mass of researchers?
Debbie: Well, funding is always an issue for all researchers. But I believe that what slows us down in MS research is our approach of studying each intervention in isolation, to make sure each is safe, one by one. That’s very slow, and doesn’t always add up to meaningful advances in the care for people with MS. Instead, I think we need to also do more of what PCORI is advocating -- to involve people with MS in defining what’s important to them, enable them to define their desired outcomes, and then do pragmatic trials to determine what’s most effective – whether an intervention or a coordinated system of care -- in helping them to achieve those outcomes. Pragmatic trials, including evaluation of systems of care, are really key. Interestingly, I see a growing awareness in the research community that such pragmatic trials may have the greatest impact in MS, and are more likely to be successful.
Q: Have you observed a change in attitudes in the research community towards patient- reported outcomes, and the overall patient role in research?
Debbie: Unfortunately, there is still pushback from both the clinical community and the research community about involving people with MS (or other conditions), since there’s a long tradition of just telling the patient what to do. Those attitudes have to change in the future, of course, and I think initiatives such as iConquerMS™ and funding mechanisms such as PCORI will play a role in such change.
Q: Why did you become involved with iConquerMS™?
Debbie: iConquerMS™ really aligns with my perspective, in that it embodies the natural integration of research and care to achieve wellness, centered around individual goals for greater function, participation and quality of life – and that’s likely to make a huge difference for people with MS. For example, it’s been the prevailing view for a long time that some people with MS will inevitably become disabled...but if you involve people in their goals for physical well-being, you may be able to empower them to do what it takes to postpone or decrease disability indefinitely. So, iConquerMS™ could be transformative for individuals as well as for cultural and intellectual change.
Q: How do you envision iConquerMS™ being of value to people with MS in the near or longer term? That is, how can a patient-driven initiative like this make a real difference?
Debbie: As iConquerMS™ evolves, I think its value becomes more apparent. It is, for sure, a platform for studies of topics that are of importance to people with MS– for example, some of the research proposals now awaiting funding range from cognitive topics to telerehabilitation. In addition, iConquerMS™ offers people with MS the opportunity to participate in research, especially for those who live in more remote areas. Finally, I think iConquerMS™ provides the setting in which we can engage and listen not only to people with MS, but to their caregivers and employers, insurers and the larger MS stakeholder group. So we may well accelerate advances much faster than traditional research has done.
Q: What does the iConquerMS™ Research Committee focus on?
Debbie: The Research Committee plays numerous roles. For example, we engage constituents in which research questions are important to them. We make sure that questions are asked in a meaningful way, and once we have results, we’ll be sure to disseminate them so that they translate at the clinical care level for impact. And, of course, we make sure to consider participant safety – both physical and emotional – in all our studies.
In the future, the Committee will be looking at the entire subject of ongoing patient engagement in research initiatives, and how other PCORI-funded networks are designing their programs. We also want to engage research investigators so that they maximize their usage of iConquerMS™ resources – the greater the critical mass, the faster we’ll make progress.
Q: Do you plan to integrate iConquerMS™ data into your own research projects?
Debbie: Absolutely! I’ve already submitted a Letter of Intent for a project in which I propose to use iConquerMS™ as a platform for surveys and dissemination of the knowledge we gain from our research.
Deborah Backus, PT, PhD, is Director of Multiple Sclerosis Research at the Shepherd Center in Atlanta, Georgia. Debbie received her B.S. in physical therapy in 1986, and her Ph.D. in neuroscience in 2004. She has combined her experiences as a physical therapist, researcher, and educator to focus on improving functional and health outcomes for people with neurological injury or disease, specifically related to people with multiple sclerosis (MS) and spinal cord injury (SCI). As part of the Eula C. and Andrew C. Carlos MS Rehabilitation and Wellness Program at Shepherd Center, she is focusing on the assessment of the health and wellness needs for people at varying stages of MS, and the evaluation of rehabilitation and exercise interventions that may be beneficial to people with MS.
The iConquerMS™ e-newsletter is published periodically to disseminate updates about this initiative, to keep the MS community informed, and to bridge those living with the disease with the research community.
Executive Editor: Marcia A. Kean
About iConquerMS™
iConquerMS™ is an initiative by and for individuals living with MS who understand the need to contribute their ideas and their health data to fuel research. It is the only MS research initiative that is a nonprofit, patient-governed, and part of a larger nationwide research network, called PCORnet. As part of PCORnet, iConquerMS™ is able to contribute health data to many research efforts, while also providing MS researchers access to data from millions of people from across the country.
For more information about iConquerMS™, visit www.iConquerMS.org, like us on Facebook at https://www.facebook.com/iConquerMS or follow us on Twitter, handle @iConquerMS.

